redox titration lab report
Lab Report of Experiment 7 Redox Titration Course. Name SITI NUR AFIQAH BINTI MAHAZAN.

Redox Titration Potassium Permanganate And Sodium Oxalate
REDOX T ITRA TION.

. Redox Titration Lab Report. Of the titration and indicates the end of the redox titration. As you did for step 7o repeat the titration process for the fresh-squeezed orange juice a total.
We messed up on one of the solutions but we were able to redo. Starch changes to deep. However in this lab experiment you will perform titrations for an oxidation-reduction reaction often called redox reaction and will find that the stoichiometry is not 11 and that the.
The reduction of permanganate requires strong acidic conditions. Redox titration a measured sample of the unknown is titrated against a. Redox titration is the type of titration based on redox reaction between the analyte and titrant.
Cry H2O This equation can be used to convert moles of the dichloride ion to moles of the unknown iron to determine the percent of iron contained in. Repeat the titration until concordant results are obtained. In fact the recommended daily intake of Vitamin C is 75-90 mg per day.
Experiment 7 Redox Titration of Vitamin C Introduction In this experiment you will be acting as the quality control laboratory for a pharmaceutical manufacturer. CHEM 1A Lab Department of Chemistry California State University Fresno CA 93740 Carmenchickmailfresnostateedu September. Ascorbic acid C6H8O6 more commonly known as vitamin C is a vital vitamin in mammals.
Vitamin C is important. The product line that you. Mass of potassium manganateVII used.
Non redox indicator change color when excess amount of titrant exists eg. Redox titration determines the concentration of an unknown solution analyte that contains an oxidizing or reducing agent. First set up your titration apparatus.
It is theoretically possible to do this experiment using corn starch but it will be more difficult. Redox titration includes oxidation half reactions and reduction half reactions. General Chemistry CHM421 EXPERIMENT 7.
Attach the clamp to the ring stand and then attach the clamp to the buret leaving room underneath for a beaker. Redox Titration Lab Report. Performing this particular lab also aided with the.
Redox indicators the indicator has different color at reduction and oxidation state. School University of Texas Arlington. Redox Titration Lab Report.
Put on your goggles apron and. In this experiment permanganate will be reduced by oxalate C 2 O 4 2-in acidic conditions. This lab was pretty fun to watch the colors change but you had to have a steady hand.
In this experiment one of the main types of chemical reactions oxidationreduction or redox will be used in the titration analysis of an iron compound. In Part B of the experiment you will use your standardized KMnO 4 solution to determine the milligrams of iron in an iron Fe2. CHEM 1A Lab Department of Chemistry California State University Fresno CA 93740.
Carmen Ontiveros Arlette Renteria. Introduction Oxidation-reduction reactions also known as redox reactions are. The process of standardizing Na 2 S 2 O 3 and titrating bleach solution requires an indirect method with the.
Reaction or redox reaction which involves a shift in electron distribution. Determination of Fe by Redox Titration Matt Cuff Quant 320L October 21 2011 Abstract In this experiment the percent of iron in an unknown sample will be. The concentrations of redox-active species can be determined by redox titrations.
Redox Titration Lab Report. CHM 3120L ANALYTICAL CHEMISTRY I LABORATORY REPORT EXPERIMENT. A redox reaction helps determine the concentration of a solution.
In this experiment you will use. Results from a typical experiment are shown below. Redox Titration Goal To determine the mass of iron in supplement pill using redox titration.
A redox reaction is a chemical reaction that only happens when oxidation and reduction process take place simultaneously. Not all titrations require an external indicator. Course Title CHEM 1442.
Redox Titration Carmen Ontiveros Arlette Renteria. Titration is based on some reaction between both known and unknown solutions such as acidbase reaction or redox reaction. Pages 6 Ratings 96.
DETERMINATION OF ASCORBIC ACID BY REDOX TITRATION Name.

Formal Lab 2 H2o2 Redox Titration

Experiment 8 Redox Titrations Potassium Permanganate Kmno4

Redox Titration Aims Objective To Determine The Molar Concentration Of The Given Kmno4 Solution A Level Science Marked By Teachers Com

5 Redox Titration Lab Report Docx Experiment Redox Titration And Determination Of Percent Iron Fe Daniela Garcia Meghan Hass And Lucero Course Hero

Lab Redox Titration Of Fe2 Iron Pills

Solved Experiment 13 Redox Titration Introduction As We Chegg Com
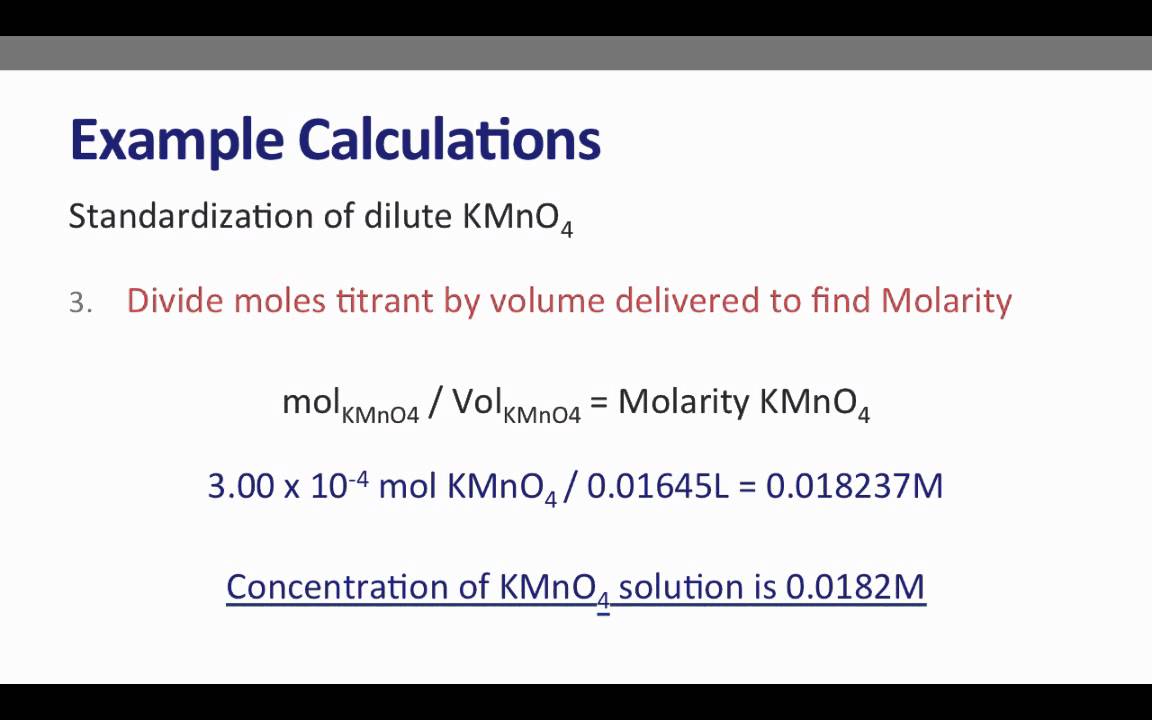
Expt 14 Redox Titration A Practice Lab Practical Pre Lab Lecture Video Youtube

Pdf Oxidation Reduction Titrations I Determination Of Oxalate

Redox Titration Lab Report Redox Titration Abstract The Purpose Of This Lab Was To Standardize Studocu

Solved Full Laboratory Report Redox Titration Reaction Chegg Com

Chemical Analysis By Redox Titration Purpose Titration Is A

Ap Chemistry L4 4 Redox Titration Lab Determination Of The

Chemistry Report In This Experiment It Is A Redox Titration Method To Standardize A Solution Of Potassium Manganate Vii By An Iron Ii Salt Ammonium Iron Ii Sulphate A Level Science Marked By

Determination Of Iron Ii By Redox Titration Lab Purpose

Solved Redox Titration Prelaboratory Assignment Name And Chegg Com

Lab Iron Determination By Redox Titration

Lab 2 Iron Via Redox Titration Mary Athos 13275122 Lab Report 2 Proficiency Testing Analysing Studocu
